- Shanghai Zhongshen International Trade Co., Ltd. - Two decades of trade agency expertise.
- Service Hotline: 139 1787 2118
As one of the major markets in North America, Canada has strict requirements and regulations for imported food. Understanding these requirements is crucial for ensuring smooth exports to Canada. This article aims to provide a basic guide on exporting food to Canada to help familiarize with relevant rules and standards.
I. Labeling Requirements
Canada has strict and detailed requirements for food labeling, especially for imported foods. Correctly understanding and applying Canadas food labeling standards is crucial for protecting consumer rights, avoiding legal risks, and successfully entering the Canadian market. Below is an introduction to Canadas food export labeling requirements.
Bilingual Labeling Requirement:Canada requires food labels to be in both English and French, which is particularly important for imported foods. All label information, including product name, ingredient list, net quantity, and shelf life, must be presented bilingually. Bilingual labeling not only reflects Canadas cultural diversity but is also a legal requirement. Therefore, ensuring every part of the label complies with this requirement is essential for smooth customs clearance and market acceptance in Canada.
Common Name:The common name of the food should accurately reflect the products actual characteristics. For example, if the food is a biscuit, its label should clearly state biscuit. Canadas Food and Drug Regulations require the common name of the product to be displayed on the principal display panel of the food label. These names should be simple and clear, avoiding misleading consumers.
Net Quantity and Font Requirements:The net quantity should be clearly indicated, usually in grams, milliliters, kilograms, or liters, depending on the products nature. There are specific requirements for the font size of the net quantity on the label. For example, the minimum font height is 1.6mm, and for larger label display panels, the font height needs to be increased further to ensure clear readability by consumers.
Ingredient List and Font Requirements:The ingredient list must be arranged in descending order by weight, except for specific ingredients that can be listed at the end. The font of the ingredient list needs to be clear and readable, typically requiring at least 1.1mm font size, and starting with bold Ingredients.,
Nutrition Facts Table:Canadian law requires that food packaging must include a Nutrition Facts Table to provide nutritional information such as energy value, protein, fat, carbohydrates, etc. The format and content of the Nutrition Facts Table must comply with specific regulations from Health Canada to ensure standardization and consistency of information.
Special Health and Safety Symbols:For foods high in sodium, sugar, and saturated fat, Canada recently requires specific magnifying glass symbols on the principal display panel to alert consumers to potential health risks. Exporters must stay updated on Canadas latest labeling regulations for specific food categories to ensure timely label updates for compliance.

II. Additive Requirements
Canada has clear regulations on which food additives can be used, along with their conditions and limits. For businesses planning to export food to Canada, understanding and complying with these additive requirements is crucial for smooth customs clearance and market acceptance.
Approval System:Health Canada oversees the approval and management of food additives. Only additives listed on the official inventory are permitted for food production and sale. Foods exported to Canada must use approved additives and comply with usage conditions and limits.
Definitions and Scope:Canada has a very clear definition of food additives, including chemical substances added during food production, processing, or storage to achieve specific technical effects. Note that traditional ingredients like salt, sugar, and nutrients such as vitamins and minerals are excluded. Processing aids are generally not considered food additives.
Functional Categories:Canada classifies food additives into multiple functional categories, such as preservatives, colorants, sweeteners, emulsifiers, etc. Exporters should thoroughly understand the functions of additives in their products and ensure compliance with usage standards and limits for the respective categories.
Lists and Standards:Health Canadas website publishes lists of food additives, each detailing which foods they can be used in and the maximum permitted levels. Exporters must carefully cross-reference these lists to ensure full compliance with all additive regulations.
Ingredient List Labeling:All additives must be listed in the ingredient list on food labels, arranged in descending order by weight. For certain additives, such as colors and sweeteners, their common names must be specifically indicated in the ingredient list.
International Standards and Acceptance:For food additives not explicitly regulated in Canada, exporters may refer to the Codex Alimentarius or other national/regional standards but must ensure Health Canada also accepts these standards. Before export, it is advisable to confirm with Canadian importers or local food regulatory agencies whether the additive usage is accepted.
Approval Process for New Additives:If an export product contains a new food additive (i.e., one not yet approved in Canada), the company must submit an application to Health Canada with detailed safety and efficacy evaluation data. This process can be time-consuming and complex, so exporters should plan ahead and prepare thoroughly.

III. Irradiated Food Requirements
Irradiation technology is widely used globally for food preservation and sterilization. Understanding and complying with Canadas specific requirements for irradiated foods is essential to ensure smooth customs clearance for exported products.
Regulatory Authorities and Legal Framework:Food irradiation in Canada is jointly regulated by Health Canadas Health Products and Food Branch (HPFB), the Canadian Food Inspection Agency (CFIA), and the Canadian Nuclear Safety Commission (CNSC). Regulations are primarily outlined in the Food and Drugs Act and the Consumer Packaging and Labeling Act, with clear specifications on irradiation procedures, permitted food types, and allowable radiation doses.
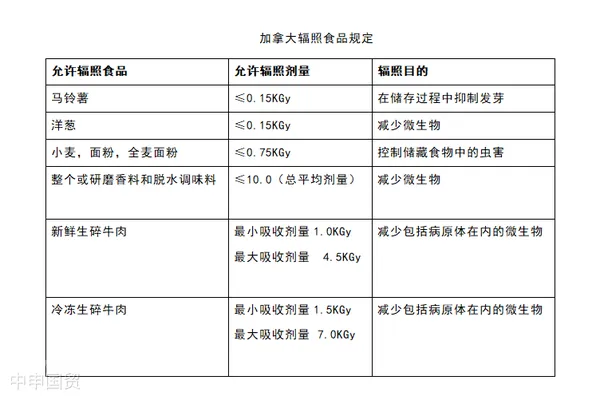
Permitted Irradiated Foods:Section B.26.003 of Division 26 in the Food and Drug Regulations lists food categories permitted for irradiation in Canada, including but not limited to potatoes, onions, wheat, flour, spices, and seasonal fresh fruits. Each food type has specific permitted irradiation doses and purposes (e.g., pest control, sprout inhibition, sterilization).
Irradiation Labeling Requirements:All prepackaged and non-prepackaged irradiated foods must clearly display irradiation information. Requirements include labeling with treated with radiation, treated by irradiation, or irradiated, or equivalent statements. For prepackaged foods, the irradiation label must be placed on the principal display panel or adjacent prominent locations. For bulk foods, irradiation information must be clearly displayed near the food counter or display area.
Irradiated Ingredients in Multi-Ingredient Foods:If irradiated ingredients constitute 10% or more of the final product, they must be explicitly labeled as irradiated in the ingredient list. If the proportion is below 10%, the irradiation declaration may be omitted.
International Uniform Irradiation Symbol:Canada requires the use of the international uniform irradiation food symbol (typically a radiation symbol with a plant pattern) to allow consumers to easily identify irradiated food. Additionally, food packaging should include detailed explanations of the irradiation symbol to address any potential consumer misunderstandings or concerns about irradiated food.

Exporting food to Canada involves more than meeting specific product and labeling requirements; companies must thoroughly understand and comply with all relevant laws and standards of the destination country. Through precise and comprehensive label management, companies can avoid customs delays and unnecessary costs due to non-compliant labeling. Ensuring that every additive used complies with Canadian regulations is essential to avoid compliance risks during import. If planning to export irradiated food, ensure full compliance with irradiation operations and labeling information. Finally, companies must stay updated on regulatory changes in Canada.
Related Recommendations
Category case
Contact Us
Email: service@sh-zhongshen.com
Related Recommendations
Contact via WeChat

? 2025. All Rights Reserved. Shanghai ICP No. 2023007705-2  PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912









